Rapid Development of Prophylactic Vaccines
Even before the outbreak of Covid-19 the Coalition for Epidemic Preparedness Innovations (CEPI) had identified rapid response platform technologies as a crucial part in the fight against future outbreaks of unknown diseases (Disease X). Using the same basic components and inserting new sequences coding for specific antigens allows for the quick adaptation of the platform for use against different pathogens. The ability to very rapidly develop a new vaccine based on a proven vaccine delivery platform has shown its worth in the recent record-breaking development of the mRNA vaccines against SARS-COV2 by Pfizer/BioNTech and Moderna.
20Med’s vaccine delivery platform is based on its non-viral bioresponsive nanoparticle technology. Specifically designed for intracellular delivery of RNA and DNA payloads the polymer-based nanoparticles are easily combined with mRNA, saRNA, or DNA coding for the relevant antigens. The manufacturing of the 20Med nanoparticles is straightforward, scalable, and independent of the payload.
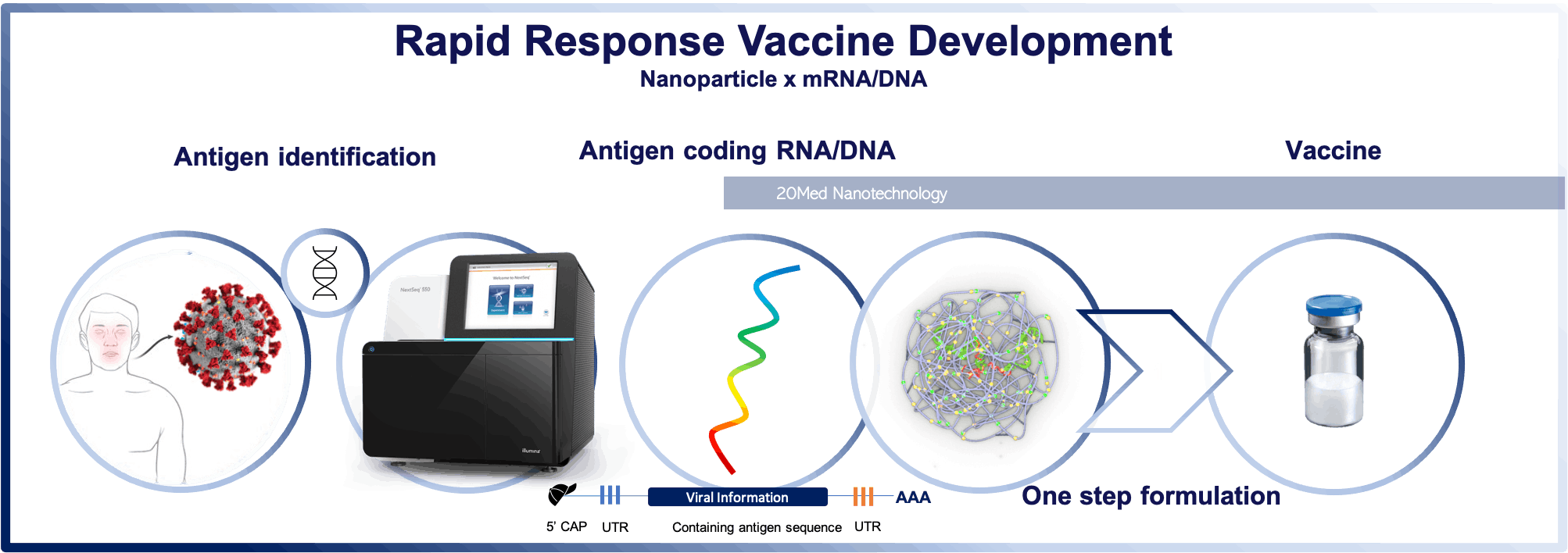
The loaded nanoparticles can be lyophilized leading to a stable vaccine product that has the potential to be stored and handled at standard refrigerator temperatures or even room temperature.
Proof of Concept Studies 20Med's Rapid Response Vaccine Platform
Proof of concept of 20Med’s vaccine development platform has been shown in the development of several RNA and DNA based vaccines against a well-known viral pathogen in animal health. 20Med nanoparticles loaded with self-amplifying mRNA coding for the pathogen’s antigens induced antibody titers similar to a viral vector-based control vaccine. Exposure of the vaccinated animals to the viral pathogen showed very good protection.
20Med nanoparticles have the potential to become the backbone of future mRNA vaccines. We have shown that novel vaccines can be developed rapidly and effectively based on the existing platform technology and that the product characteristics of the nanoparticles are very favorable for scale-up of production, ease of formulation, and stability resulting in a lyophilized thermostable final product.
Partnering
If you are interested in discussing the application of the 20Med nanoparticle vaccine platform for your vaccine development efforts, please contact us at BD@20medtx.com or send us an inquiry through the contact page